First of all, Nitrous Oxide is not a fuel.
Nitrous is not flamable, thus, the scene which Brian shouts in Fast & the Furious "NOOsSSS!!" and the green Mitsubishi explodes is not impossible.
Using the term "NOS" is completely wrong, because NOS is the name of a company, just like MOMO, SPARCO or APEXI. The right term would be "Nitrous Oxide".
Nitrous Oxide}
Nitrous oxide, also known as dinitrogen oxide or dinitrogen monoxide, is a chemical compound with chemical formula N2O. Under room conditions, it is a colourless non-flammable gas, with a pleasant, slightly-sweet odor. It is commonly known as laughing gas due to the exhilarating effects of inhaling it, and because it can cause spontaneous laughter in some people. It is used in surgery and dentistry for its anaesthetic and analgesic effects. Nitrous oxide is present in the atmosphere where it acts as a powerful greenhouse gas.
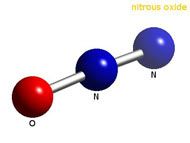
The structure of the nitrous oxide molecule is a linear chain of a nitrogen atom bound to a second nitrogen, which in turn is bound to an oxygen atom.
Nitrous oxide N2O should not be confused with the other nitrogen oxides such as nitric oxide NO and nitrogen dioxide NO2.
Nitrous oxide can be prepared by heating ammonium nitrate in the laboratory.
>
Nitrous oxide can be used to produce nitrites by mixing it with boiling alkali metals, and to oxidize organic compounds at high temperatures.
The CAS number of nitrous oxide is 10024-97-2 and its UN number is 1070.
The gas was discovered by Joseph Priestley in 1772. Humphry Davy in the 1790s tested the gas on himself and some of his friends, including the poets Samuel Taylor Coleridge and Robert Southey. They soon realised that nitrous oxide considerably dulled the sensation of pain, even if the inhaler were still semi-conscious. And so it came into use as an anaesthetic, particularly by dentists, who do not typically have access to the services of an anesthesiologist and who may benefit from a patient who can respond to verbal commands.
Nitrous Oxide Tuning
In car racing, nitrous oxide (often just "nitrous" in this context) is sometimes injected into the intake manifold (or just prior to the intake manifold) to increase power: even though the gas itself is not flammable, it delivers more oxygen than atmospheric air by breaking down at elevated temperatures, thus allowing the engine to burn more fuel and air. Additionally, since nitrous oxide is stored as a liquid, the evaporation of liquid nitrous oxide in the intake manifold causes a large drop in intake charge temperature. This results in a smaller, denser charge, and can reduce detonation, as well as increase power available to the engine.
The same technique was used during by World War II Luftwaffe aircraft with the GM 1 system to boost the power output of aircraft engines. Originally meant to provide the Luftwaffe standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialized planes like high-altitude reconnaissance aircraft, high-speed bombers and high-altitude interceptors.
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to destroy the engine. Power increases of 100-300% are possible, and unless the mechanical structure of the engine is reinforced, most engines would not survive this kind of operation.
There are several ways of introducing nitrous into a motor. Nitrous kits such as NOS, Nitrous Express, Nitrous Direct brands offer different solutions. You will find Dry kits, Wet kits & Direct port.
It is very important with nitrous oxide augmentation of internal combustion engines to maintain temperatures and fuel levels so as to prevent preignition, or detonation (sometimes referred to as knocking, pinging or pinking).