Converting CO2 in ethanol isn’t a new concept but, up until now, it hasn’t exactly been easy. During an experiment at the Oak Ridge National laboratory in Tennessee, scientists stumbled across a new method that is that uses common materials and nanotechnology to create ethanol from Co2. The experiment was initially designed to determine a series of chemical reactions that would make the conversion, but much to the surprise of everyone working on the project, the very first step in a series of many produced the result they were looking for.
To put things simply, copper and carbon are arranged into nanospikes on a silicon surface, ultimately allowing the reaction create to be very precise with a limited number of contaminants – essentially reversing the combustion process. Dr. Adam Rondinone – one of the authors of this study – said, “By using common materials, but arranging them with nanotechnology, we figured out how to limit the side reactions and end up with the one thing that we want. A process like this would allow you to consume extra electricity when it's available to make and store as ethanol. This could help to balance a grid supplied by intermittent renewable sources.”
Outside of the fact that the whole process can be done with common, and therefore cheap, materials, the most important aspect is that the reaction that creates the ethanol can be created at room temperature. This means that the process can be controlled easily and will little energy cost. And, while it can have major positive implications for the automotive industry – cars are said to be a major contributor to the on-going CO2 and global warming problem – it could really help bring peace to the entire energy industry as this process is a controllable way to make fuel efficiently.
Renewable energy sources like wind and solar power are growing in popularity, but we have no way to control the weather and therefore cannot control when we generate energy from these sources. This process isn’t exactly the solution to our global energy needs quite yet, but it could ultimately provide the buffer needed for us to rely more on renewable energy in the future.
Keep reading for the rest of the story.
Making the Difficult Easy… By Accident
The men behind this experiment have openly said that this new process was stumbled on by accident, but it was the ultimate goal of the experiment – they just got the results they wanted in the first step.
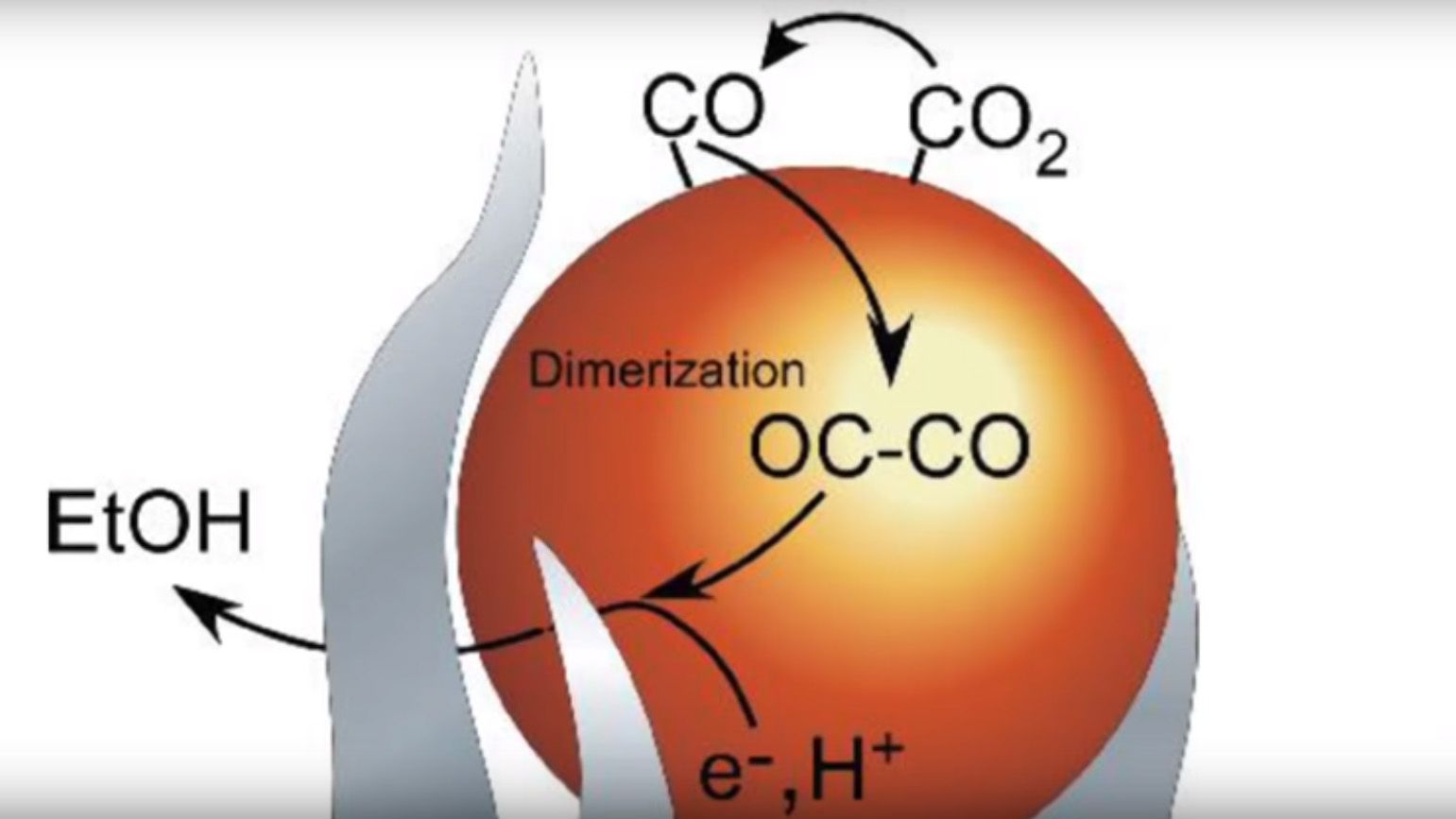
“We’re taking carbon dioxide, a waste product of combustion, and we’re pushing that combustion reaction backward with very high selectivity to a useful fuel,” Rondinone continued, “Ethanol was a surprise -- it’s extremely difficult to go straight from carbon dioxide to ethanol with a single catalyst.”
All the team had to do was use a catalyst made of carbon, copper, and nitrogen, and then apply voltage to a trigger. This, somehow, reverses the combustion process and after the CO2 is dissolved in water, it turns into ethanol with an average yield of about 63 percent. To put that into perspective, this kind of reaction normally creates a handful of different products in small amounts.
What it Means for the Auto Industry
As already mentioned before, this in no way solves the global energy crisis, but it could have a positive effect on the automotive industry. A lot of newer vehicles have the capability of running E85, a fuel blend that is composed of anywhere between 51- and 83-percent ethanol depending on the location and season. Could it be possible to automate the process and scale it down enough to allow it to be built into future vehicles? If so, we could harness the CO2 emissions created by modern internal combustion engines and turn that into fuel as we drive – ultimately increasing overall fuel economy reducing the CO2 emissions that your gas-guzzling all-American SUV spews into the atmosphere every time you drive. Of course, this is just speculation, and it would require out vehicles to carry a source of nitrogen and copper at all times, but it’s a thought. The researchers aren’t stopping with this new development, as they will continue to work in an attempt to increase the efficiency of this new conversion process even further.
You can read the full paper on the process by visiting Chemistry Select.